Note
This page was generated from scgen_perturbation_prediction.ipynb. Some tutorial content may look better in light mode.
scGen - Perturbation response prediction¶
This tutorial reproduces https://scgen.readthedocs.io/en/stable/tutorials/scgen_perturbation_prediction.html with our custom Jax implementation.
[1]:
import pertpy as pt
import scanpy as sc
from scvi import REGISTRY_KEYS
ryp2 is not installed. Install with pip install rpy2 to run tools with R support.
Loading Train Data¶
[2]:
train = pt.dt.kang_2018()
[3]:
train.raw = train.copy()
sc.pp.normalize_total(train)
Let’s remove stimulated CD4 T cells from the training set, as we want to generalize our stimulated condition to them.
[4]:
train_new = train[~((train.obs["cell_type"] == "CD4 T cells") & (train.obs["label"] == "stim"))]
train_new = train_new.copy()
Preprocessing Data¶
[5]:
pt.tl.SCGEN.setup_anndata(train_new, batch_key="label", labels_key="cell_type")
Creating the model¶¶
[6]:
scgen_model = pt.tl.SCGEN(train_new)
WARNING: All log messages before absl::InitializeLog() is called are written to STDERR
I0000 00:00:1700241529.542823 1 tfrt_cpu_pjrt_client.cc:349] TfrtCpuClient created.
Training the Model¶
[7]:
scgen_model.train(
max_epochs=100,
batch_size=32,
early_stopping=True,
early_stopping_patience=25,
accelerator="cpu",
)
GPU available: True (mps), used: False
TPU available: False, using: 0 TPU cores
IPU available: False, using: 0 IPUs
HPU available: False, using: 0 HPUs
Epoch 100/100: 100%|██████████| 100/100 [1:15:03<00:00, 44.92s/it, v_num=1, train_loss_step=6.41e+3, train_loss_epoch=3.06e+4]
`Trainer.fit` stopped: `max_epochs=100` reached.
Epoch 100/100: 100%|██████████| 100/100 [1:15:03<00:00, 45.04s/it, v_num=1, train_loss_step=6.41e+3, train_loss_epoch=3.06e+4]
Saving the model¶
[8]:
scgen_model.save("../saved_models/model_perturbation_prediction.pt", overwrite=True)
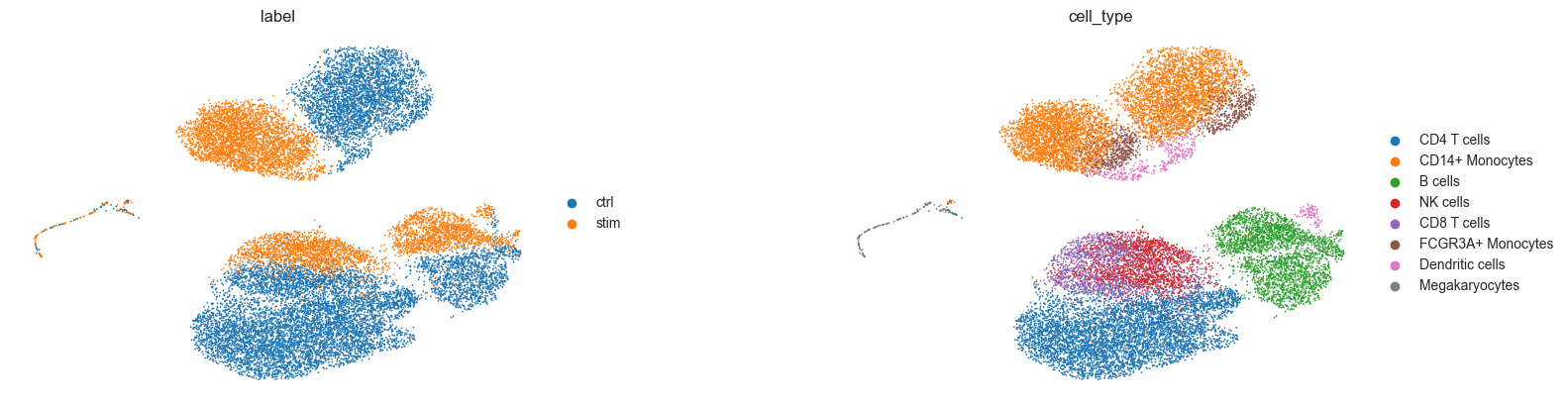
Latent Space¶
We will now obtain the latent representation that was calculated by our model and visualize it using UMAP.
[9]:
latent_X = scgen_model.get_latent_representation()
latent_adata = sc.AnnData(X=latent_X, obs=train_new.obs.copy())
[10]:
sc.pp.neighbors(latent_adata)
sc.tl.umap(latent_adata)
sc.pl.umap(
latent_adata,
color=["label", "cell_type"],
wspace=0.4,
frameon=False,
save="latentspace_batch32_klw000005_z100__100e.pdf",
)
WARNING: You’re trying to run this on 100 dimensions of `.X`, if you really want this, set `use_rep='X'`.
Falling back to preprocessing with `sc.pp.pca` and default params.
WARNING: saving figure to file figures/umaplatentspace_batch32_klw000005_z100__100e.pdf
Prediction¶
After training the model you can pass the adata of the cells you want to perturb. Here we pass unperturbed CD4 T cells
Here the AnnData object contains the cells that you want estimate the perturbation based on them. we set “ctrl” to our control labels and “stim” to our stimulated labels. If you apply it in another context just set “ctrl” :”your_control_label” and “stim”:”your_stimulated_label”. the returned value is a numpy matrix of our predicted cells and the second one is the difference vector between our conditions which might become useful later.
[11]:
pred, delta = scgen_model.predict(ctrl_key="ctrl", stim_key="stim", celltype_to_predict="CD4 T cells")
pred.obs["label"] = "pred"
INFO Received view of anndata, making copy.
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
INFO Received view of anndata, making copy.
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
INFO Received view of anndata, making copy.
INFO Input AnnData not setup with scvi-tools. attempting to transfer AnnData setup
Evaluation of the prediction¶
Extracting both control and real stimulated CD4 T cells from our dataset¶
[12]:
ctrl_adata = train[((train.obs["cell_type"] == "CD4 T cells") & (train.obs["label"] == "ctrl"))]
stim_adata = train[((train.obs["cell_type"] == "CD4 T cells") & (train.obs["label"] == "stim"))]
Merging predicted cells with real ones
[13]:
eval_adata = ctrl_adata.concatenate(stim_adata, pred)
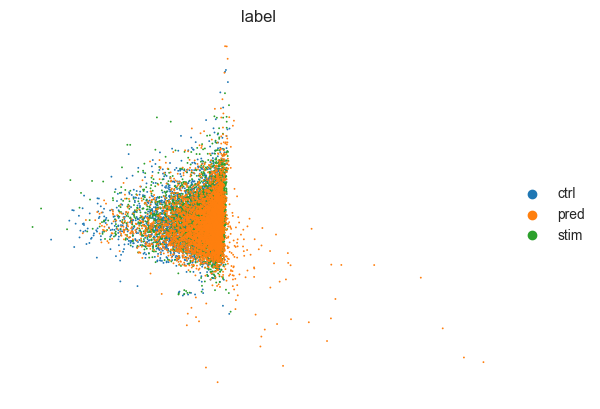
Embedding all real and predicted cells in one PCA plot¶
[14]:
sc.tl.pca(eval_adata)
sc.pl.pca(
eval_adata,
color="label",
frameon=False,
save="pred_stim_b32_klw000005_z100__100e.pdf",
)
WARNING: saving figure to file figures/pcapred_stim_b32_klw000005_z100__100e.pdf
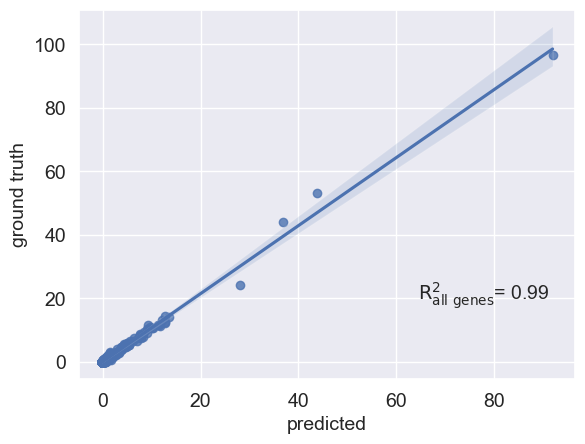
Mean correlation plot¶
You can also visualize your mean gene expression of your predicted cells vs control cells while highlighting your genes of interest (here top 10 differentially expressed genes)
[15]:
CD4T = train[train.obs["cell_type"] == "CD4 T cells"]
[16]:
sc.tl.rank_genes_groups(CD4T, groupby="label", method="wilcoxon")
diff_genes = CD4T.uns["rank_genes_groups"]["names"]["stim"]
print(diff_genes)
WARNING: It seems you use rank_genes_groups on the raw count data. Please logarithmize your data before calling rank_genes_groups.
['ISG15' 'IFI6' 'ISG20' ... 'EEF1A1' 'FTH1' 'RGCC']
[17]:
condition_key = scgen_model.adata_manager.get_state_registry(REGISTRY_KEYS.BATCH_KEY).original_key
r2_value = scgen_model.plot_reg_mean_plot(
eval_adata,
condition_key=condition_key,
axis_keys={"x": "pred", "y": "stim"},
labels={"x": "predicted", "y": "ground truth"},
path_to_save="./reg_mean.pdf",
show=True,
legend=False,
)
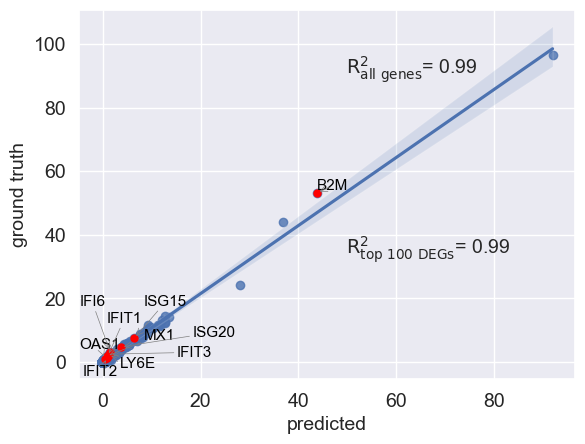
You can also pass a list of differentially expressed genes to compute correlation based on them
[18]:
r2_value, r_value_diff = scgen_model.plot_reg_mean_plot(
eval_adata,
condition_key=condition_key,
axis_keys={"x": "pred", "y": "stim"},
gene_list=diff_genes[:10],
top_100_genes=diff_genes,
x_coeff=0.91,
y_coeff=0.76,
labels={"x": "predicted", "y": "ground truth"},
path_to_save="./reg_mean_diff_genes.pdf",
show=True,
legend=False,
)
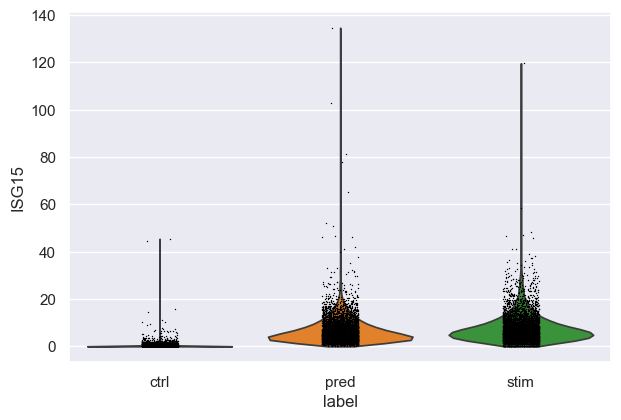
Violin plot for a specific gene¶
Let’s go deeper and compare the distribution of “ISG15”, the top DEG between stimulated and control CD4T cells between predcited and real cells:
[19]:
sc.pl.violin(eval_adata, keys="ISG15", groupby="label")